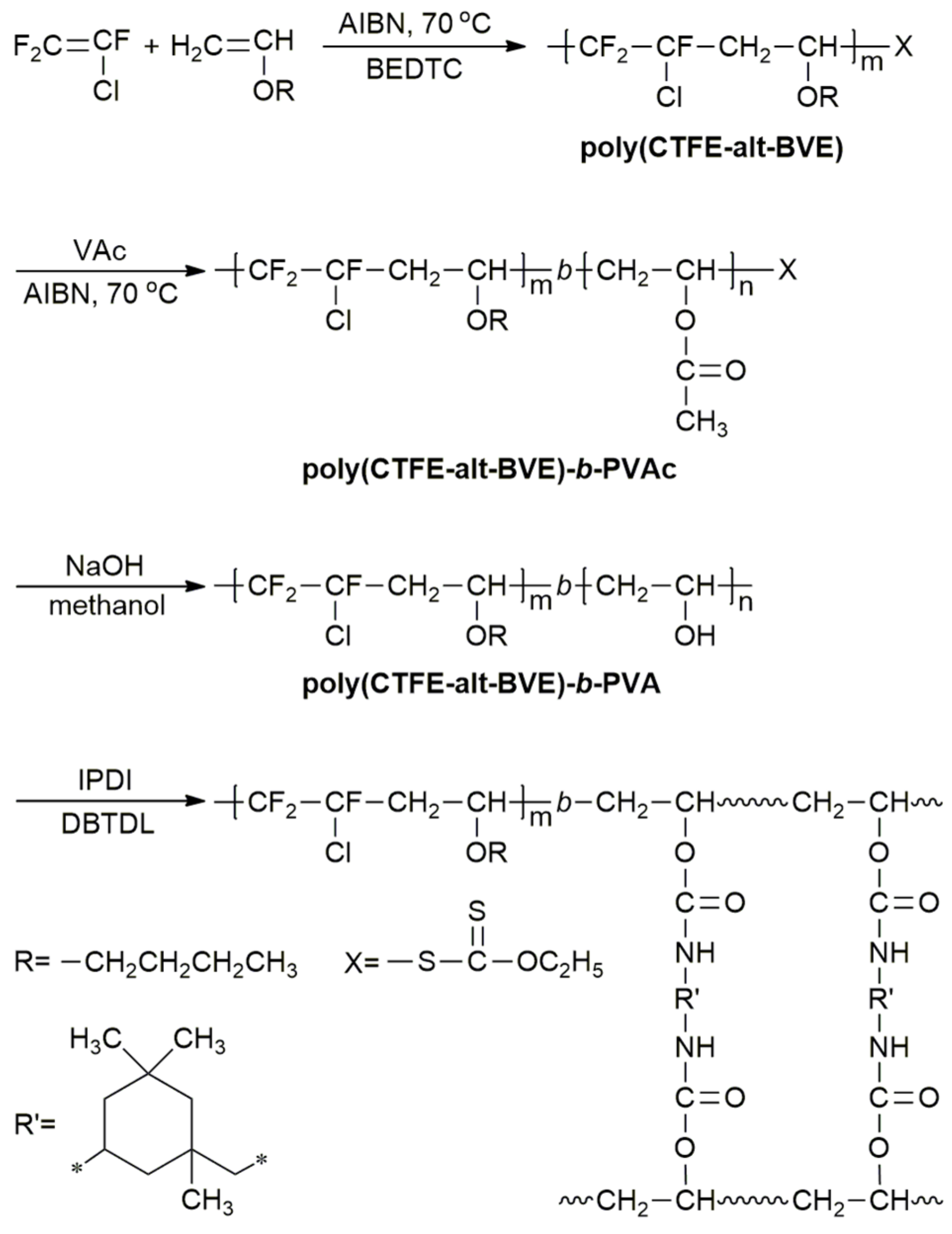
By fine tuning the ethylene pressure and the vinyl acetate content a broad range of copolymers containing from 0 to 85 mol of vac unit was achieved.
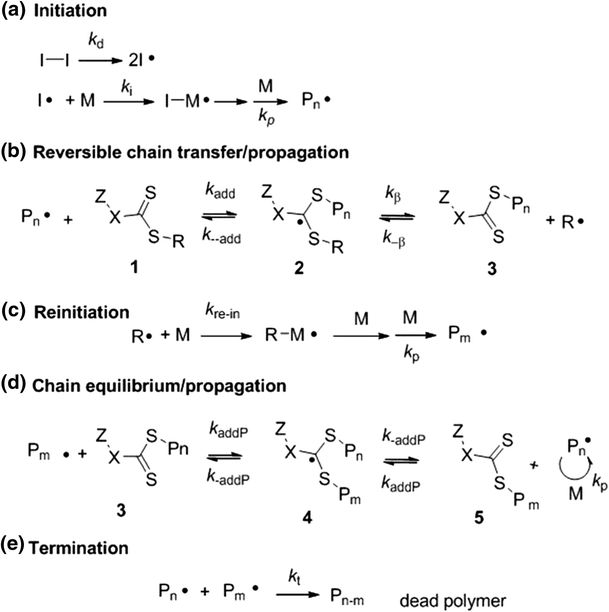
Ethylene vinyl acetate polymerization mechanism.
Eva copolymers are commercially used predominantly in the areas of coating laminating and in the film industries.
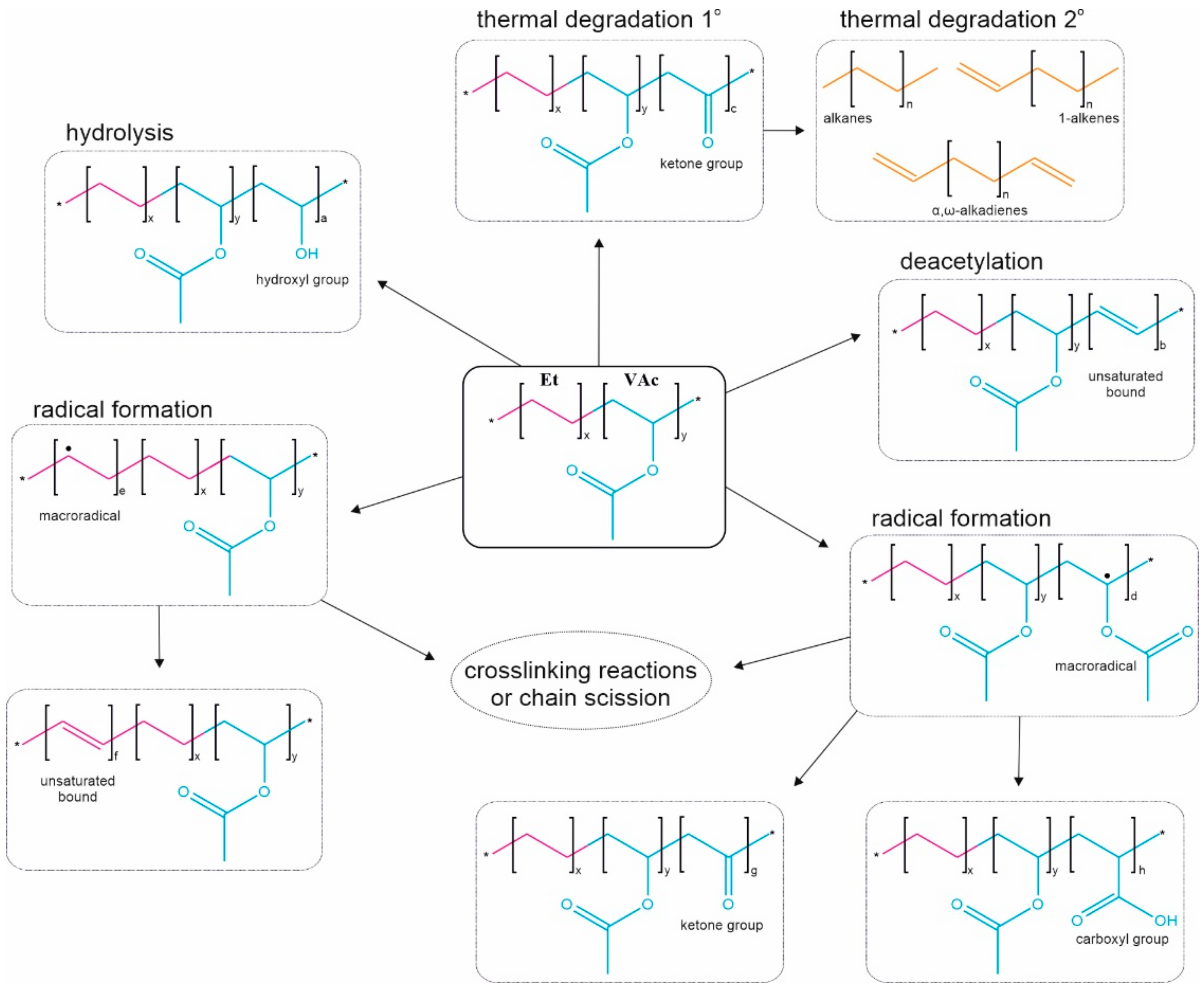
In the production of ethylene vinyl acetate copolymers in a free radical catalyst initiated high pressure process explosive decomposition is prevented by contacting the reactor effluent downstream.
This method involves the gas phase addition of acetic acid to acetylene in the presence of metal catalysts.
Us2703794a us245079a us24507951a us2703794a us 2703794 a us2703794 a us 2703794a us 245079 a us245079 a us 245079a us 24507951 a us24507951 a us 24507951a us 2703794 a us2703794 a us 2703794a authority us united states prior art keywords ethylene vinyl acetate weight copolymer water prior art date 1951 09 04 legal status the legal status is an assumption and is not a legal conclusion.
The effects of temperature pressure added co solvent vinyl acetate feed rate and emulsifier type and concentration on the rate of polymerization cumulative copolymer composition molecular.
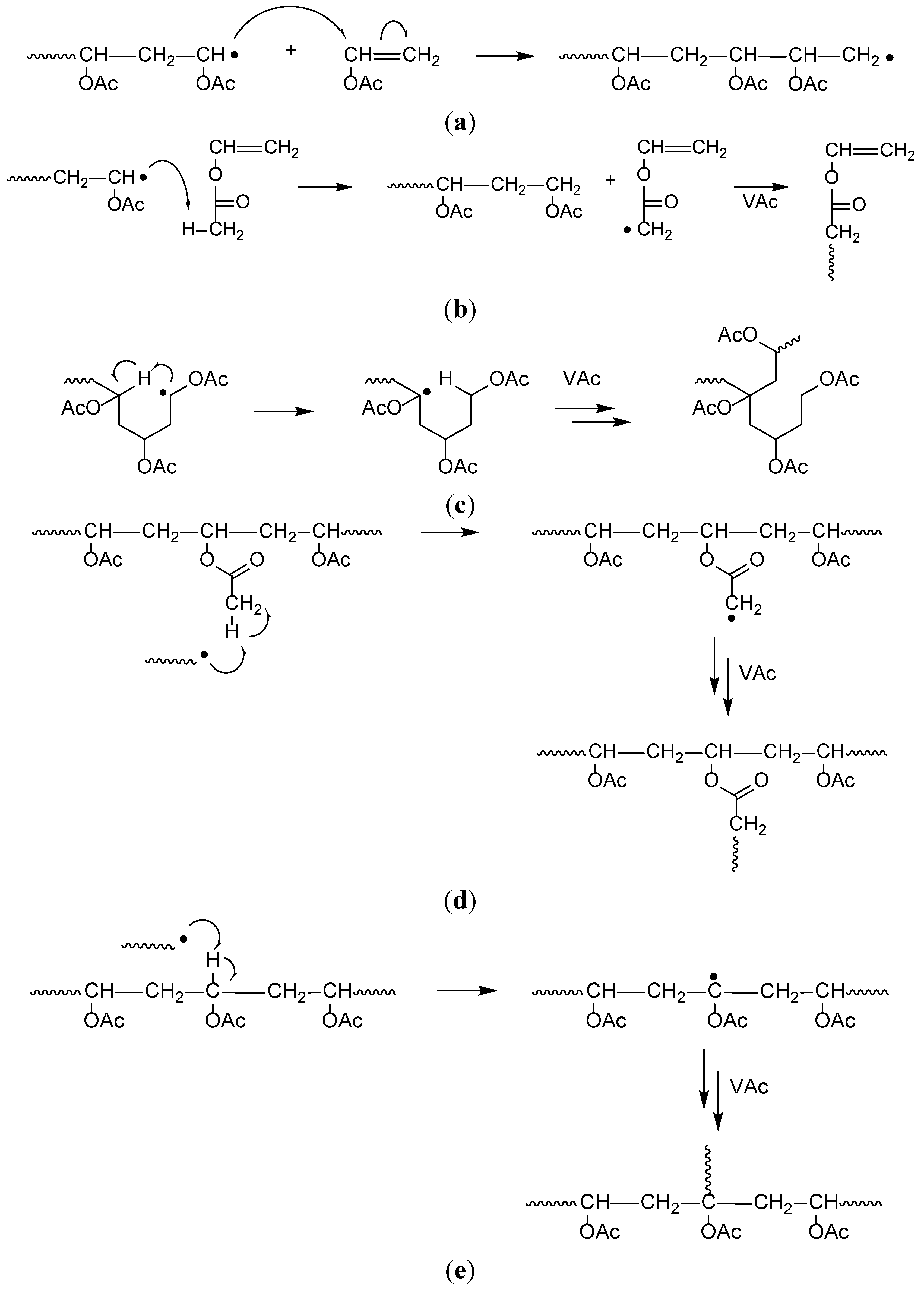
The polymerization reaction is initiated by forming alkene metal complex.
There are three different types of eva copolymer which differ in the vinyl acetate va content and the way the materials are used.
When a vinyl monomer like propylene comes to the active metal center it can be coordinated to ti atom by overlapping their orbitals.
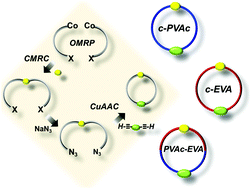
Coordination copolymerization of vinyl acetate vac with ethylene leading to linear copolymers that possess in chain ch 2 ch oac units has been accomplished using novel palladium complexes bearing alkylphosphine sulfonate ligands.
The copolymerization by itp itcop of ethylene with vinyl acetate vac to form poly ethylene co vinyl acetate eva copolymer was also successful.
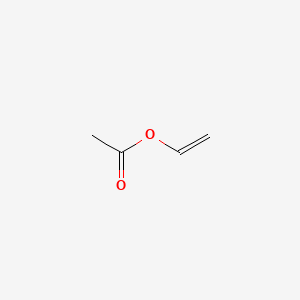
Eva generally contains 1 50 of the va comonomer along the carbon chain backbone.
Therefore vinyl acetate runaway polymerization incidents are very serious and they occur with a high frequency.
The polymerization of vinyl acetate is probably the second most frequent cause of runaway reaction accidents in the chemical industry after the phenol formaldehyde runaway reaction.
Ethylene vinyl acetate eva also known as poly ethylene vinyl acetate peva is the copolymer of ethylene and vinyl acetate the weight percent of vinyl acetate usually varies from 10 to 40 with the remainder being ethylene.
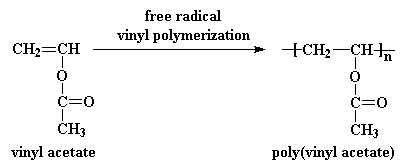
Vinyl acetate was once prepared by hydroesterification.




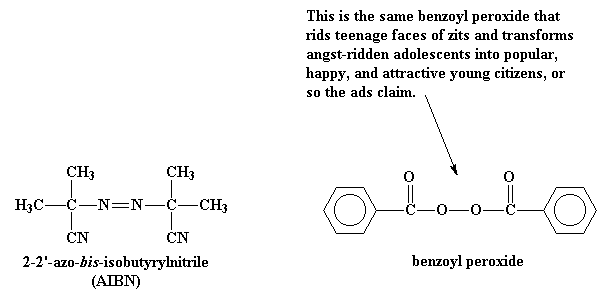
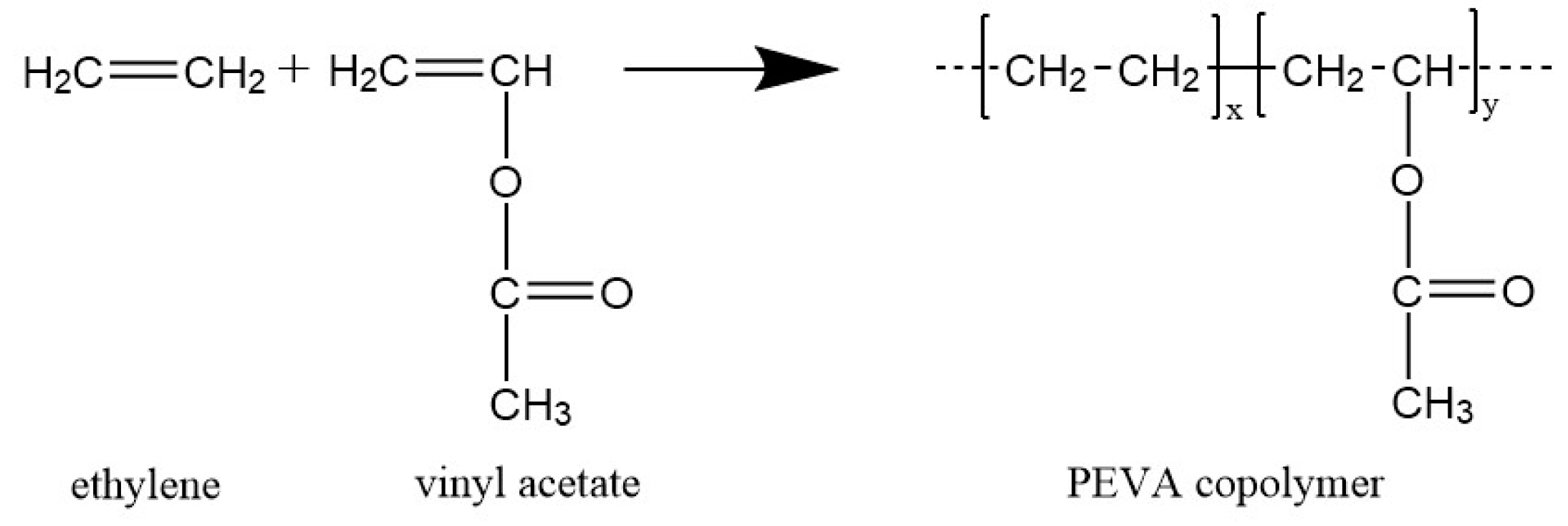

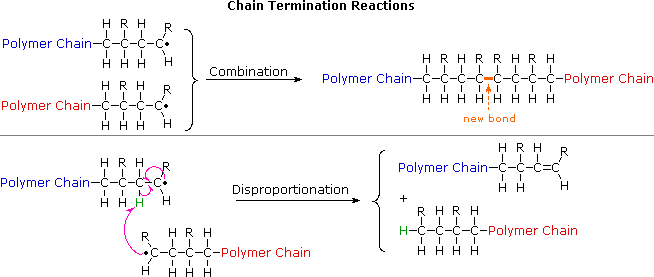




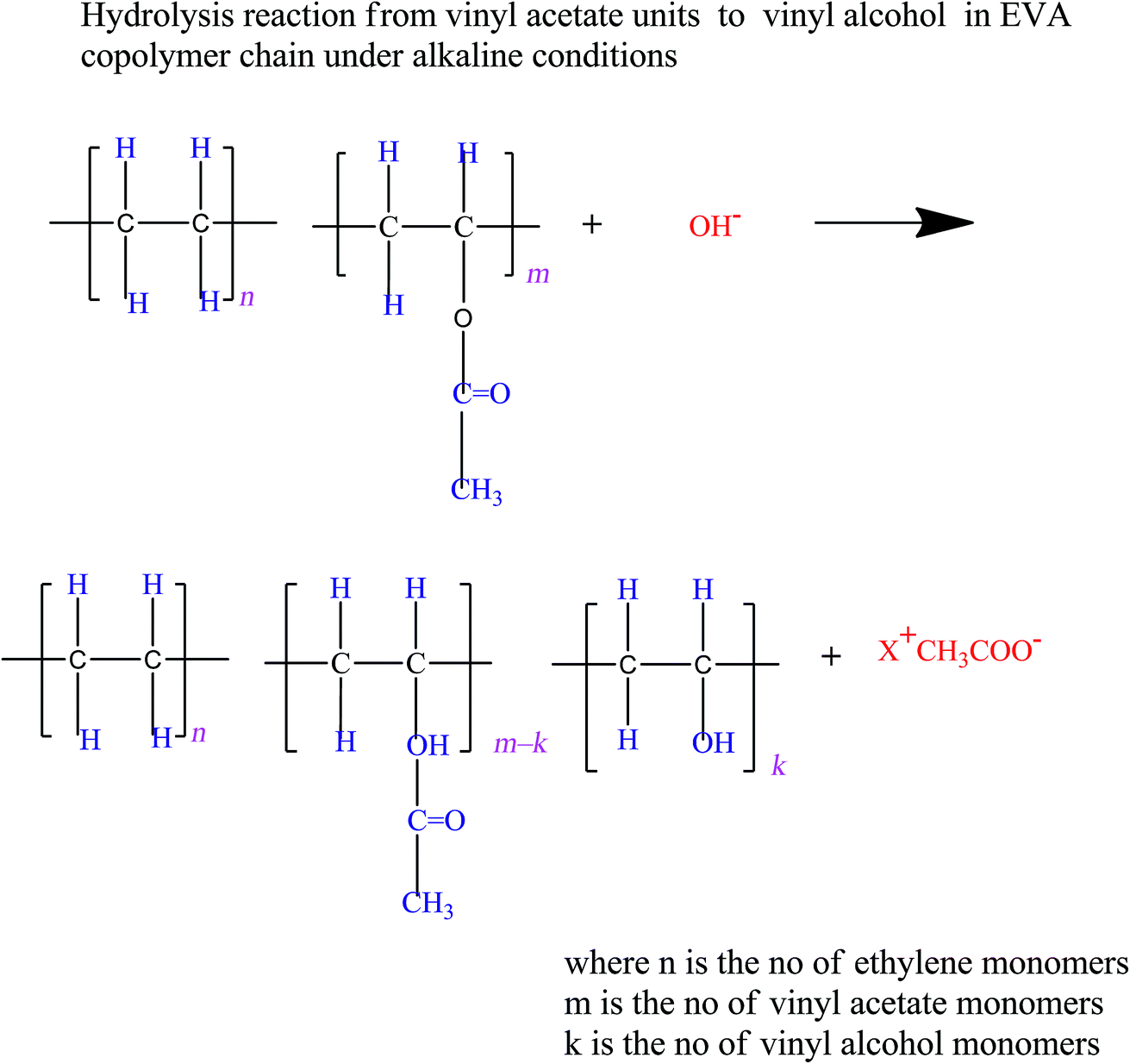
.jpg)